Dr Sean Xie is Founding Director of CCGS Center at UPitt. He also serves as an Associate Dean for Research Innovation and a PI/Professor of an integrated medicinal chemistry biology laboratory of CompuGroup, BioGroup and ChemGroup (www.CBLigand.org/xielab) and a charter member of the Science Advisory Board to the US FDA. He served as co-PI and core Director on two NIH-funded center grants (PCMLD NIH P50 and UP-CDC NCI/SAIC) and served as co-PI on NIH PMLSC grant. Recently, Dr Xie has led as Director/PI of the NIH-funded National Center of Excellence for Computational Drug Abuse Research (CDAR) (www.CDARCenter.org), a joint “Big-Data” and computational initiative between the University of Pittsburgh and Carnegie Mellon University.
CCGS Center has its research on target identification and system pharmacology for drug lead/chemical probe discovery and cell signaling mechanism studies, with over 100 publications and invention discovery patents. The innovative work was achieved by our developed GPU-accelerated cloud computing TargetHunter machine-learning programs and diseases-specific chemogenomics knowledgebase. Such a platform of “Big data to knowledge” (www.CBLigand.org/CCGS) was built on our extensive knowledge and solid working experience in development of know-how technologies and application of in silico design and virtual screening, computational chembiology, medicinal chemistry optimization, and biophysics/biochemistry validation for pharmacometrics system pharmacology and translational drug discovery research. Our recent work on Alzheimer’s disease specific chemogenomics database was on 2014 coverpage story of a top peer-reviewed ACS journal. The innovation work includes GPU-accelerated cloud computing TargetHunter program for drug target identification (www.CBLigand.org/TargetHunter) (2013 AAPS special theme issue). As such, we received the 2014 AAPS Award for Outstanding Research Achievements.
In addition, we have acquired expertise in bone, blood and immune-origin cancer chemical biology research, including our discovery of a first p62-ZZ chemical inhibitor for multiple myeloma (published two patents and 2015 Leukemia), a first p18-targeted chemical inhibitor for hematopoietic stem cell expansion (a patent and Nature Comm, 2015), and cannabinoid CB2-targeted osteoporosis and immune research (patents, JMC and PNAS). Particularly, our discovered p62ZZ chemical agents and two invention discovery patents were licensed to Biotech/Pharma companies for further translational research.
Key Technologies:
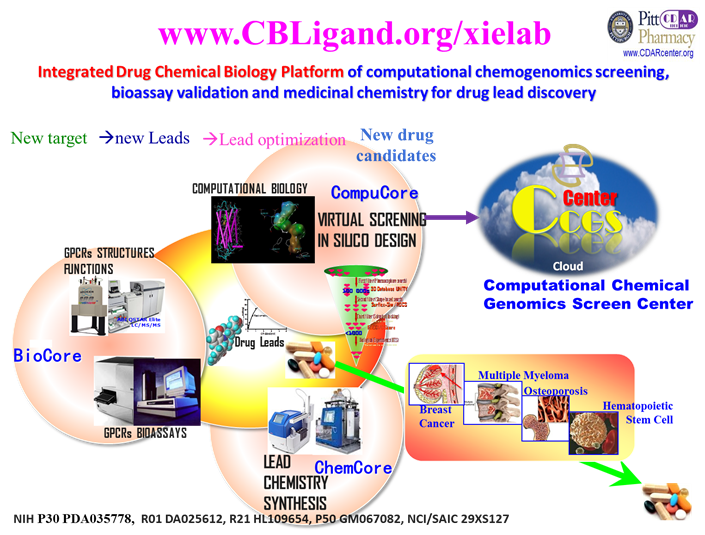
An Integrated Drug Chemical Biology Platform – computational chemogenomics screening, bioassay validation, medicinal chemistry and system pharmacology for drug lead discovery and development.
 Selected Outcomes (Xie as a senior correspondent): Selected Outcomes (Xie as a senior correspondent):
- Gao Y, Yang P, Shen HM, Yu H, Xie ZJ, Zhang L, Bartlow P, Ji Q, Ding Y, Wang L, Liu H, Ma H, Hao S, Dong F, Li Y, Zhang P, Cheng H, Liang PH, Miao W, Yuan Y, Cheng T* and Xie XQ* “Small-molecule inhibitors targeting INK4 protein p18INK4C enhance ex vivo expansion of haematopoietic stem cells”, Nature Communication, 2015 Feb 18;6:6328. PubMed PMID: 25692908.
- Teramachi J, Rebecca S, Yang P, Zhao W, Mohammad K, Guo JX, Anderson HL, Zhou D, Feng R, Myint KZ, Maertz N, Beumer JH, Eiseman, JL, Windle JL, Xie XQ*, Roodman D*, Kurihara N*. “Blocking the ZZ Domain of Sequestosome1/p62 Suppresses Myeloma Cell Growth and Osteoclast Formation In Vitro and Induces Dramatic New Bone Formation in Myeloma-Bearing Bones In Vivo” Leukemia (Nature) 2015, Aug 19. (doi: 10.1038/leu.2015.229) PMID:26286116 (*Senior co-correspondents)
- Liu, HB, Wang, L, Su, WW and Xie XQ*, ALzPlatform: An Alzheimer’s Disease Domain-Specific Chemogenomics Knowledgebase for Polypharmacology and Target Identification Research. J Comput Info Modeling.2014, 54(4):1050-60. (Coverpage of the issue).
- Wang L, Ma C, Wipf P, Liu H, Su W and Xie XQ*. “TargetHunter: An In Silico Target Identification Tool for Predicting Therapeutic Potential of Small Organic Molecules Based on Chemogenomic Database”. AAPS J. 2013, 15, 395-406 (AAPS Special theme issue)
- Ma, C, Wang, LR, Xie, XQ* “GPU Accelerated Chemical Similarity Calculation for Compound Library Comparison” JCIM, 51 (2011), 1521–1527. Ma C, Wang LR, Yang P, Tong Q, Myint KZ, and Xie X-Q*. “LiCABEDS II. Modeling of Ligand Selectivity for G-protein Coupled Cannabinoid Receptors”, J. Chem. Inf. Model., 2013, 53(1):11-26
KEY RESEARCH PROJECTS
1. GPU-accelerated cloud computing TargetHunter machine-learning programs and diseases domain-specific chemogenomics knowledgebase for target identification system pharmacology drug discovery and cell signaling mechanism studies
Xie’s lab is known for its pioneering research for the development of diseases domain-specific chemogenomics knowledgebase database, an integrated platform of target identification, system pharmacology and translational research. These include Alzheimer’s, Drug Abuse, Cardiovascular, Osteoporosis, Multiple Myeloma, Stem Cells, Diabetes databases (www.cbligand.org/CCGS/research.php). His recent work on Alzheimer’s disease specific chemogenomics knowledgebase represents an innovative “data to knowledge” system accessible at www.CBLigand.org/AD/, and has won 2014 coverpage story of a top ACS peer-reviewed journal1. His lab is in process to develop HD (Huntington), PD (Parkinson) and TBI (Trauma Brain Injury) specific chemogenomiocs database (b-version available), implemented with his developed computational algorithms/tools, including the GPU-accelerated2 TargetHunter© program for drug target identification (2013 AAPS special theme issue, www.CBLigand.org/TargetHunter)3, machine learning Ligand Classifier of Adaptively Boosting Ensemble Decision Stumps (LiCABES) algorithms for GPCR ligand selectivity or functionality prediction (JCIM 2012, 2013)4, 3D chemistry-space matrix-based compound library acquisition and prioritization (CLAP) profiling algorithm for constructing/purchasing structural-diverse chemical libraries (2013 ACS Comb. Science coverpage), and molecular fingerprint-based artificial neural network algorithm for QSAR analysis, HTDocking, and BBB-predictor (www.cbligand.org/xielab/technology.php.
- Liu, HB, Wang, L, Su, WW and Xie* X.-Q., ALzPlatform: An Alzheimer’s Disease Domain-Specific Chemogenomics Knowledgebase for Polypharmacology and Target Identification Research. J Comput Info Modeling.2014, 54(4):1050-60. (Coverpage of the issue).
- Ma, C, Wang, LR, Xie, XQ* “GPU Accelerated Chemical Similarity Calculation for Compound Library Comparison” JCIM, 51 (2011), 1521–1527
- Wang L, Ma C, Wipf P, Liu H, Su W and Xie X-Q*. “TargetHunter: An In Silico Target Identification Tool for Predicting Therapeutic Potential of Small Organic Molecules Based on Chemogenomic Database”. AAPS J. 2013, 15, 395-406 (AAPS Special theme issue)
- Ma C, Wang LR, Yang P, Tong Q, Myint KZ, and Xie X-Q*. “LiCABEDS II. Modeling of Ligand Selectivity for G-protein Coupled Cannabinoid Receptors”. J. Chem. Inf. Model., 2013, 53(1):11-26
- Xie X-Q*, Wang L, Liu H, Ouyang Q, Fang C and Su W. “Chemogenomics Knowledgebased Polypharmacology Analyses of Drug Abuse Related G-Protein Coupled Receptors and Their Ligands”. Front. Pharmacol.(Neuropharmacology), 2014, 5:3.
2. First INK4C/p18 chemical modulators for hematopoietic stem cell (HSC) expansion
Xie’s Lab was the first to discover and report (Nature Comm 2015)1 a small molecule that inhibits p18INK4C (or p18), a stem cell regulator protein and member of the INK4 family of cyclin-dependent kinase (CDK) inhibitor proteins, and demonstrated that the compound stimulates HSC expansion in murine and human.
Hematopoietic stem cells (HSC) are multipotent adult stem cells that give rise to all the blood cell types including myeloid (monocytes and macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes/platelets, dendritic cells), and lymphoid lineages (T-cells, B-cells, NK-cells). However, broader use of HSC is hindered by the limited number of HSC per harvest. Although HSC expansion can be achieved by ectopic expression of several positive regulators via retrovirus or lentivirus-mediated methods, these viral approaches are unfortunately associated with a higher risk of leukemogenesis. Other approaches, including transgenesis, cytokines, new protein factors, stromal cells, and bioreactors, have also been tested to expand HSCs, but the specificity for HSCs and the efficacy of these approaches in clinical trials have not been determined. Indeed, alternative novel approaches for HSC expansion are still in critical need.
We have developed an integrated computational and experimental chemogenomics-based approach for discovery and development of novel p18-targeted chemical promoters of HSC self-renewal that overcomes the difficulty of performing costly and time-consuming conventional biochemical studies with adult stem cells that are extremely rare in tissues. Thus, using our integrated methods2-4, we have discovered the first p18 small molecule inhibitors (p18SMI) and validated the bioactivities (ED50 = 3.07 μM) by our established in vitro and in vivo HSC experiments in collaboration with Dr. Tao Cheng, a former faculty at UPCI. We subsequently confirmed the p18 target specificity by performing the Cobblestone-Area Forming Cell (CAFC) assay using long-term (LT) cultures of wild type (WT, p18+/+) and knockout (p18-/-) murine mouse bone marrow (mBM) cells. Importantly, p18 target specificity of p18SMIs was also confirmed by a [g-32P] CDK6 bioactivity assay and co-IP pull down assay. With lead optimization medicinal chemistry synthesis, newly synthesized p18SMIs exhibited significantly improved HSC expansion bioactivity with ED50 in low nM range. Finally, we confirmed that p18SMIs promoted expansion of both murine and human HSCs ex vivo by a serial competitive bone marrow transplantation (cBMT) model, a “gold standard” in vivo functional HSC measurement. Notably, the p18SMIs did not show significant cytotoxicity toward myeloblast-like 32D cells or primary HSCs, nor did it augment myeloma cell proliferation. Taken together, our newly discovered p18SMIs represent novel chemical agents for murine and human HSCs ex vivo expansion and also can be used as valuable chemical probes for further HSC biology research towards promising utility for therapeutic purposes.
- Gao Y, Yang P, Shen HM, Yu H, Xie ZJ, Zhang L, Bartlow P, Ji Q, Ding Y, Wang L, Liu H, Ma H, Hao S, Dong F, Li Y, Zhang P, Cheng H, Liang PH, Miao W, Yuan Y, Cheng T* and Xie XQ* “Small-molecule inhibitors targeting INK4 protein p18INK4C enhance ex vivo expansion of haematopoietic stem cells”, Nature Communication, 2015 Feb 18;6:6328. PubMed PMID: 25692908;
- Xie XQ*, Invited speaker, Nov 2012, "Novel Small Chemical Inhibitors Targeting p18INK4C Protein for Hematopoietic Stem Cell Expansion”, 3rd International Forum on Stem Cells, Tianjin, China
- Yang P., Zhang P., Zhang Y., Wang L., Tong, Q., Cheng T., Gao Y., and Xie X.Q*. “Novel p18INK4C small molecule inhibitors for hematopoietic stem cell expansion”. ACS 2014 Central Regional Meeting. Oct, 2014, Pittsburgh, USA
- Cheng T, Xie X, Gao Y (Patent) "p18 small molecule chemical inhibitor and human hematopoietic stem cell expansion ex vivo”. Patent No. 201510081641.Publication No: 2015061001183450. Classification No. C07C303/38(2006.01) (300457) 2015-06-12 17:27. Xie XQ* et al, Stem Cell Chemogenomics Knowledgebase, 2013, http://www.cbligand.org/stemcell
3. First p62Z chemical inhibitor with therapeutic potentials
Xie’s lab was the first to discover and develop p62ZZ chemical inhibitors with promising anti-multiple myeloma (MM) therapeutic index (Nature Leukemia 2015 in press) in collaboration with Dr. David Roodman. Actually, emerging studies show that regulatory roles of p62 in protein-protein interactions and autophagy have recognized p62 as a validated therapeutic target for the treatment of cancer and neurodegenerative diseases. Our innovation is based on the therapeutic knowledge and our data that the Sequestosome-1 (or p62) plays critical roles in the survival, growth and metastasis of MM cells. P62 is a key domain activating NF-kB, and p38 MAPK, both of which are aberrantly activated in MM, thus it is a promising drug target for MM treatment. We have proved that the ZZ domain of p62 is responsible for increased MM cell growth and osteoclast (OCL) formation mediated by NF-kB and p38 MAPK signaling.
Our discovered lead compound XIE3P62ZZ (#3 or XRK3, 4.31 mM) exhibited notable p62 antagonistic effects and significantly reduced survival of human MM cells and also inhibited osteoclastogenesis (Patent: Xie et al. PCT/US2012/049911). The specificity was further confirmed by p62-/- experiments, in which effects of the inhibitor XRK3 on MM cells and tumor necrosis factor (TNF)-α-induced osteoclast (OCL) formation were lost upon p62-/- gene deficiency. Our preliminary chemistry modification of this lead produced more potent p62 inhibitor analogs (e.g., XIE1-10b: IC50 1.12, XIE1-62a: 0.63 mM) with improved drug-like properties (t1/2 = 4.5 hrs, Bioavailability 43%). The in vivo MM xenograft murine model revealed significantly inhibited MM tumor growth (>75%) and increased mean survival time (53%) compared with the control group. We have also demonstrated that: · NF-κB and p38 MAPK signaling are increased in primary bone marrow stromal cells from MM patients; · genetic silencing or deletion of p62 in patient- or mouse-derived stromal cells resulted in a marked reduction in the capacity of stromal cells to support the growth of MM cells and osteoclastogenesis; · the p62 ZZ-type zinc finger domain was identified to be the structural basis of p62 to support osteoclastogenesis and the growth of MM cells. · p62-ZZ domain specificity of XRK3 is confirmed by co-IP pull down assay. The discovered p62Z chemical inhibitors will be valuable chemical probe for studies of cell signaling pathways associated with diseases such as multiple myeloma.
- Teramachi J, Rebecca S, Yang P, Zhao W, Mohammad K, Guo JX, Anderson HL, Zhou D, Feng R, Myint KZ, Maertz N, Beumer JH, Eiseman, JL, Windle JL, Xie XQ*, Roodman D*, Kurihara N*. “Blocking the ZZ Domain of Sequestosome1/p62 Suppresses Myeloma Cell Growth and Osteoclast Formation In Vitro and Induces Dramatic New Bone Formation in Myeloma-Bearing Bones In Vivo” Leukemia (Nature) 2015, Aug 19. (doi: 10.1038/leu.2015.229) PMID:26286116 (*co-correspondents).
- Xie, X-Q*, Myint, K. Kurihara N, Roodman, D.: “P62-ZZ Chemical Inhibitor for Multiple Myeloma Intervention” (2012), USSN 61/521,287, PCT/US2012/049911. Xie X-Q: “New provisional patent application No. 62/174,465: “p62-ZZ CHEMICAL INHIBITOR”. Filed on June 11, 2016
- Teramachi J, Myint KZY, Feng RT, Xie XQ, Windle JJ, Roodman D, Kurihara N. “Blocking the ZZ Domain of Sequestosome 1/p62 Suppress the Enhancement of Myeloma Cell Growth and Osteoclast Formation by Marrow Stromal Cells”. Blood, 2011, 118(21):406.
4. Design and discovery of novel cannabinoid receptor CB2 ligands
The cannabinoid receptor CB2 is highly expressed in immune cells and is an important G-protein coupled receptor (GPCR) for immune signal transduction. Emerging studies in mice and humans suggest an important role for the endocannabinoid system in autoimmune, inflammation, and immune-origin diseases. Thus, targeting CB2 receptor is a promising therapeutic strategy for the treatment of these diseases, particularly for osteoporosis (a bone morphogenetic protein auto-immune disorder), multiple myeloma (MM) (immune B plasma caused bone disease). Dr. Sean Xie and his team have discovered novel CB2 chemical agents with promise for treatment of multiple myeloma and osteoporosis diseases. We have designed and synthesized a series of selective CB2 inverse agonists with novel chemical scaffolds, and top 3 compounds showed excellent CB2 binding affinity (Ki < 10 nM, selectivity index > 100) and effective anti-osteoporosis and anti-MM activities. Our innovative CB2 compounds exhibit good drug-like properties with minor toxicity.
- Four Patents: (1) Xie* X-Q, Chen J, Zhang Y: "Ligands specific for cannabinoid receptor subtype 2". PCT/US2008/012395; WO 2009/058377; US20110118214 A1. (2) Xie* X-Q, Feng R, Yang P: “Novel cannabinoid receptor 2 (CB2) inverse agonists and therapeutic potential for multiple myeloma and osteoporosis bone diseases”. USSN 61/576,041, 2012.
- Yang P, Myint KZ, Tong Q, Feng R, Cao H, Almehizia AA, Wang L, Bartlow P, Gao Yand Xie XQ*: “Lead discovery, chemistry optimization, and biological evaluation studies of novel biamide derivatives as CB2 receptor inverse agonists and osteoclast inhibitors”. J Med Chem2012, 55(22):9973-9987.
- Yang P, Wang L, Feng R, Almehizia AA, Tong Q, Myint KZ, Ouyang Q, Alqarni MH, Xie XQ*: “Novel triaryl sulfonamide derivatives as selective cannabinoid receptor 2 inverse agonists and osteoclast inhibitors: discovery, optimization, and biological evaluation.” J Med Chem2013, 56(5):2045-2058.
- Ouyang Q, Tong Q, Peng RT, Myint KZ, Yang P, Xie XQ*: “Trisubstituted Sulfonamides: A New Chemotype for Development of Potent and Selective CB2 Receptor Inverse Agonists.” ACS Medicinal Chemistry Letters2013, 4(4):387-392.
5. In vitro bioassay and in vivo animal tudies of osteoporosis and other bone diseases related
Xie has established innovative computational-chemogenomics-knowledgebase-guided CB2-mediated signaling mechanism studies and system pharmacology research as well as in vitro osteoclast and in vivo ovariectomized (OVX) rat osteoporosis model experiments through collaboration with D Roodman and D Gilson (see their letters). Due to the interactions between myeloma cells and cells of the bone marrow microenvironment, the osteoporosis (OP) or osteolytic bone disease associated with myeloma is inextricably linked with tumor progression. High incidence of bone metastasis in MM patients (95-100%) is associated with severe bone pain and pathological fracture (60%), which has been defined as the result of activated osteoclast and suppressed osteoblast. With many years of developed research investigations and how-know technologies, we have found that high levels of expression of active CB2 were observed in human MM cell lines and primary CD138+ myeloma cells. Our discovered CB2 ligand, phenylacetylamide (PAM, or XIE95), inhibits MM growth through cell cycle modulation, mitotic death and cytoskeleton disruption. In particular, our studies show that CB2 gene silencing rescues PAM-induced inhibition of MM cell proliferation. Furthermore, it’s been reported that CB2 is expressed in osteoclast and osteoblast as well as their precursor cells. CB2-/- mice showed accelerated age-related trabecular bone loss and cortical expansion. CB2-/- osteoclast number and trabecular osteoblast activity were increased. The role of CB2 pathway is important in MM-induced bone remodeling. We found that our compound favorably suppressed osteoclast formation in both murine and human osteoclastogenesis. Our top CB2 compounds demonstrated promising therapeutic index by animal efficacy and toxicity laboratory studies.
- Feng R, Tong Q, Xie Z, Wang L, Lentzsch S, Roodman GD, Xie XQ*: Targeting cannabinoid receptor-2 pathway by phenylacetylamide suppresses the proliferation of human myeloma cells through mitotic dysregulation and cytoskeleton disruption. Mol Carcinog2015:PMID: 25640641
- Feng RT, Milcarek CA, Xie XQ*: Antagonism of cannabinoid receptor 2 pathway suppresses IL-6-induced immunoglobulin IgM secretion. BMC Pharmacol Toxico2014, 15:30 PMID:24913620.
- Gertsch, J.; Leonti, M.; RadunerS.; RaczI.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.; Zimmer, A.; Karsak, M. Beta-Caryophyllene is a Dietary Cannabinoid. PNAS 105(2008), 9099-9104.
|